Growers are facing increased regulation, especially when it comes to the application of nitrogen. Nitrogen takes a lot of energy to produce, has negative environmental impacts and is expensive. As a result, there’s a growing interest in reducing fertilizer use — because YOU CAN. If farms can start paying more attention to biological nitrogen fixation for crop production, they can realize comparable yields with less input.
Plant Available Nitrogen
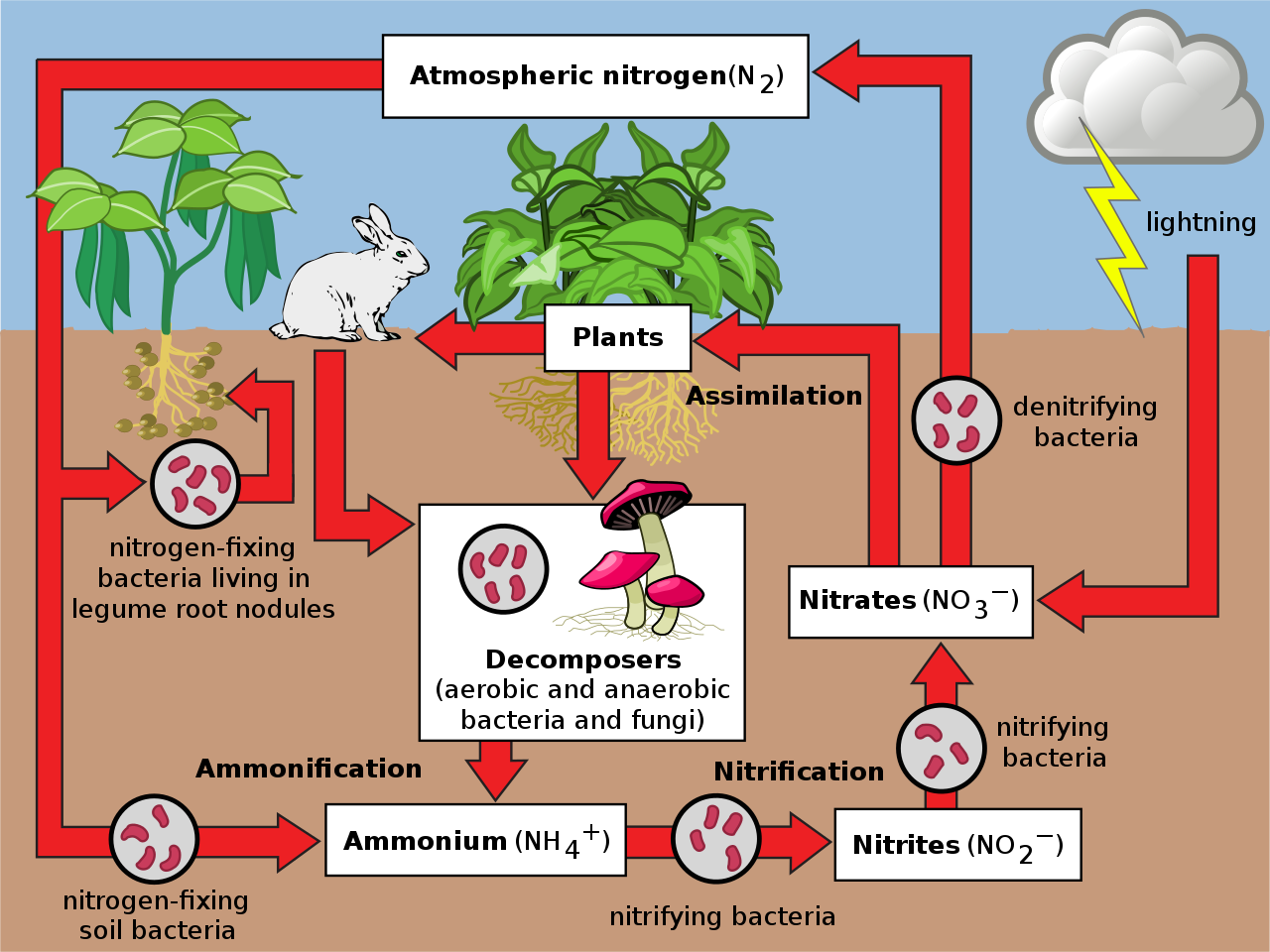
The nitrogen cycle is dependent on microbiology. Although almost 80% of our atmosphere is nitrogen, it’s in a form (N2) that can’t be utilized by plants. N2 gas is a very stable compound due to the strength of the triple bond between the nitrogen atoms, and it requires a large amount of energy to break this bond. In order to make nitrogen available to plants, we need the help of bacteria. They consume atmospheric nitrogen and secrete ammonium (NH4+). This energetically demanding process of converting N2 into biologically available nitrogen is called nitrogen fixation, and only a select group of bacteria and cyanobacteria are able to carry it out.
Once this initial conversion takes place, ammonium can be consumed by other bacteria that produce nitrite (NO2-). Finally, another kind of bacteria eats nitrite and secretes nitrate (NO3-). All of these forms of nitrogen are available to the plant, but nitrate is the easiest for plants to access. The major take-away is that we must have a robust level of bacteria in our soils to make nitrogen available to the plant. It’s foundational to successful farming.
Nitrogen Fixers
Peas, beans and clover are included in the more than 18,000 species in the pea family. Most species in this family are known as nitrogen fixers, as they increase the level of nitrogen in the soil, which all plants need to produce proteins and chlorophyll. Members of the pea family can be planted with your cash crops or planted as cover crops, to produce more nitrogen in the soil. Nitrogen fixing plants don’t produce nitrogen themselves, they create a habitat for the nitrogen-fixing microbes. Clover, for example, has little nodules that house huge numbers of bacteria. The ammonium that these bacteria create is slowly released into the soil. When the plant dies, the bacteria disperse into the soil and result in an abundance of plant allies available to convert even more nitrogen.
Other organisms are closely involved. Fungi, attached to the plants’ roots, expand the plants’ ability to access the nitrogen and bring it in to be used by the plant. The plant, in turn, exudes sugars and carbohydrates to feed the fungi. Worms eat dead plants and bring the material down into the rhizosphere where they poop out food which is a delicacy for the growing bacteria. We know this process as decomposition.
Nitrogen Volatilization, Nitrification, and Denitrification
Nitrogen exits the soil through harvest or when water carries it away. It can also heat up and become gaseous, returning to the atmosphere in what is called volatilization. Or, if the soil is dying and lacks oxygen, different anerobic bacteria grow and covert nitrates back into N2, what is called denitrification. These losses of nitrogen only happen with loose nitrogen in the soil, not with nitrogen inside organisms. The transformation of nitrogen into its many oxidation states is key to productivity in the biosphere and is highly dependent on the activities of a diverse assemblage of microorganisms, such as bacteria, archaea, and fungi.
Synthetic Nitrogen Degrades Soils
Synthetic nitrogen isn’t processed by the plants and ends up in waterways or sensitive environments, spurring regulation. Nitrogen inputs mostly remain in the loose soil and are prone to leaching, versus being incorporated into micro-organisms where it stays put. Loose nitrogen is also much more prone to volatilization, which produces nitrous oxide, a greenhouse gas. Quantities of pure nitrogen irritate earthworms and they end of dying or moving. It disrupts the helpful fungi on plant roots. It changes the pH of soil, making it inhospitable to bacteria. In short, it kills the soil so now you have to add more nitrogen because there is aren’t any organisms in the soil to do the work for the plant. The root fungi also supply important micronutrients, so now you need to add micronutrients. You end up spending more and more money on fertilizer when you could have taken advantage of all the nature provides!
The process of making synthetic fertilizers for use in agriculture by causing N2 to react with H2, known as the Haber-Bosch process, has increased significantly over the past several decades. In fact, today, nearly 80% of the nitrogen found in human tissues originated from the Haber-Bosch process (Howarth 2008).
As human populations continue to increase, the consequences of human activities continue to threaten our resources and have already significantly altered the global nitrogen cycle. The good thing is that we have viable options for improvement that deliver a host of benefits.
Andaman Ag carries a large variety of microbial products from TwinN nitrogen fixing bacteria, to MetaGrow Compost Teas, Pacific Gro Oceanic Fish Hydrolysate, Tainio Biologicals, Phyto-Catalysts, Ecotech Biological fertilizers, Fertum Seaweed extracts, and many more. Including these materials with current practices can enable growers to produce better crops for less.